customers' demand
The effect of any administered therapeutic is mainly determined by the concentration that it reaches at the target site (1). If a newly developed drug, for instance against Alzheimer's Disease, Cancer, Coronary heart (& stroke), Crohn's Disease and Diabetes mellitus, which are among the TOP 10 causes of death with around 31 million events/year (2), does not reach the target area with sufficient concentration, its curing efficacy can be expected to be insignificant.
Hence, an imaging method that could provide a quantitative in-vivo tracking of therapeutics, is of high impact. Apart from such a next-generation pharmacokinetics, which would allow the quantitative localization of administered medical drugs, one could use the same methodology for localizing medical diagnostic agents. For instance, such agents could target immune cells for monitoring inflammations. Another option is to label immune cells in such a way that different types can be distinguished in-vivo.
These unprecedented imaging capabilities for a faster & more effective market access of medical innovations is highly interesting to reduce the average costs of up to 2,7 billion USD of drug development, reduce the average timeline of 13 years till market launch and increase at the same moment ROI due to limited patent time of 20 years.
- J. Vrbanac, R. Slauter, in A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition), 2017
- World Health Organization. Fact Sheet No. 310. http://www.who.int/mediacentre/factsheets/fs310/en
our solution
Basics
our solution is based on the advancement of current X-ray fluorescence imaging (XFI) - IP-protected methodologies - owned by axiom insights.
For visibility medical drugs or diagnostic agents are bound on XFI markers, such as gold nanoparticles or other elements, or cells are loaded by them.
These XFI markers, that can be excited to emit "X-ray echos" (i.e. X-ray fluoresence) and by recording these "echos" the nanoparticles are localized ex vivo and in vivo.
Chemical elements between Zirconium and Neodymcan can be used as XFI markers, so they can be chosen such that they do not affect the entities.
In details
You can see in the figure, an X-ray pencil beam from the synchrotron scans along cells which are loaded with XFI makers such as gold nanoparticles (GNPs). A photon (red line) from the X-ray beam can remove an inner shell electron from a maker atom. This empty electron position is subsequently filled with an electron from a higher atomic shell. As a result of this event, an X-ray photon, the "X-ray echo", is emitted into an arbitrary direction (orange line) where it can be recorded as a signal.
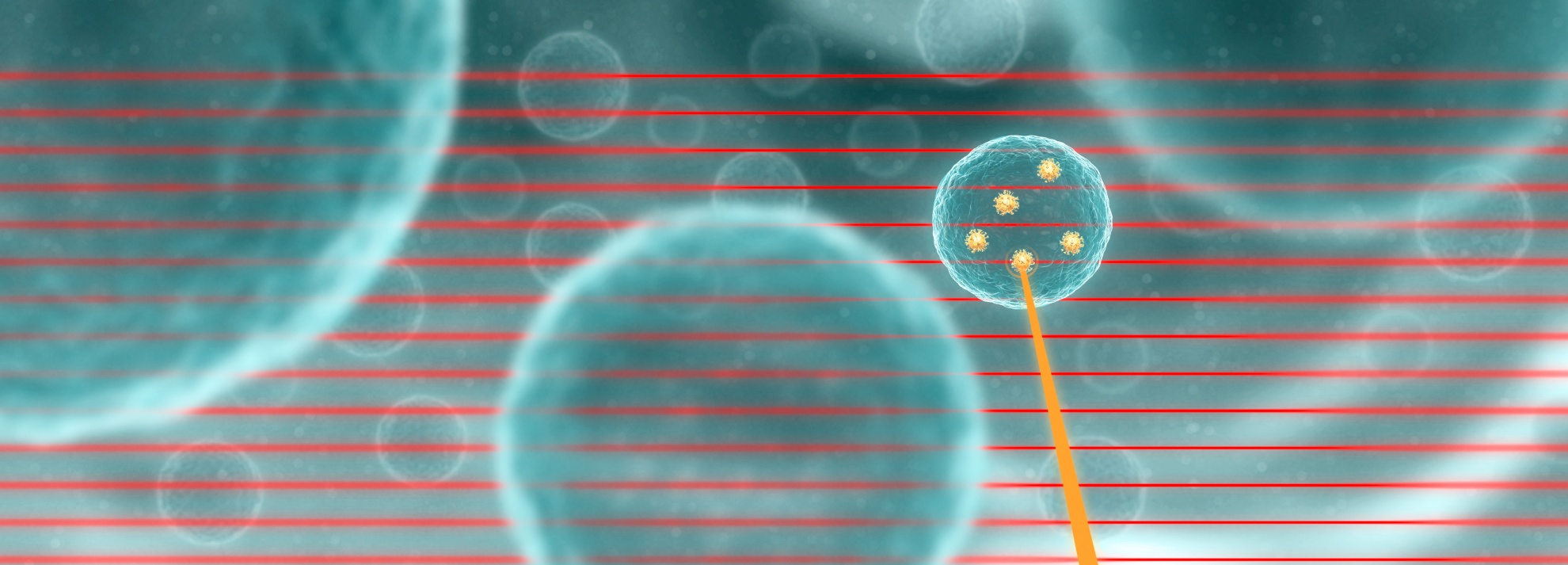
Backround of advanced XFI from axiom
X-ray Fluorescence Imaging is already known, but could not be implemented for measurements in larger objects until now. The reason? X-rays are scattered numerous times inside the body. This leads to a disruptive background, from which the actual X-ray signals of the fluorescent markers (XFI markers), which are linked to the entity being measured, cannot not be read out. The UHH has solved this problem with a patent-protected method, which axiom has acquired and thus activated XFI as a new supplier of data in pharmaceutical research. The method provides excellent spatial resolution for ex vivo (in situ) and in vivo measurements without depth restrictions or time limitations, and is highly sensitive.
See our publication and proof of concept here.
How is the method used in praxis?
Advanced XFI method from axiom needs high energy and brilliant x-ray light sources.
At Deutsches Elektronen-Synchrotron (DESY), the large-scale research facility in Hamburg (Germany), we have access to one of the world's most brilliant X-ray light sources (PETRA-III synchrotron beamlines) to do our measurements and analytics.
Data gained
Since XFI is a scanning modality, the data gained is depicted as a map (typically on a two-dimensional projection plane). Each pixel refers to the amount of XFI-markers located in this scan beam direction. The scan time for such a map depends on the size of the target area, resolution, and required sensitivity.
If you require a full 3D map, we can apply XFI-CT or -tomosynthesis mode, however, this would require longer exposure time and a higher dose. Two-dimensional projection maps are usually sufficient because the objects of interest such as immune cells, are concentrated at the sites of interest. This means that the diffuse background does not overlay these areas and that it can be subtracted when using just a few different scanning angles (tomosynthesis).
Visualisation
axiom insights maps typically have a spatial resolution of 1.0 mm and a temporal resolution on the scale of minutes. We can also repeat the scans at different time points to deliver 4D maps.
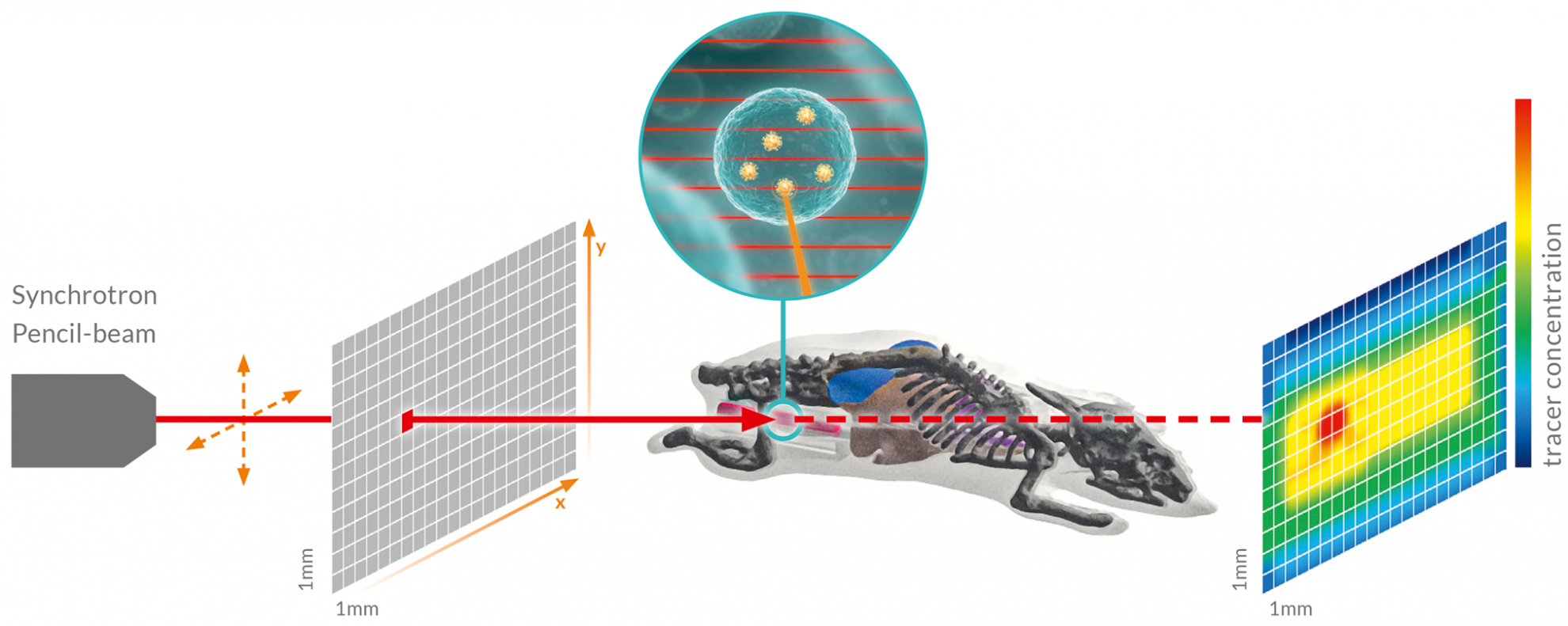
In the figure you can see an example of small animal in vivo tracking scheme. When an XFI marker is hit by the beam, a scanning X-ray pencil beam (red) from the DESY PETRA-III synchrotron facility excites an “X-ray echo”, i.e. fluorescence. Scanning typically takes place across a projection plane (grey). For each scanning direction (red square), the concentration of the marker retrieved is depicted in a “heat map” (color map) after data analysis.
Value for customers
This offers several aspects which the pharma industry, biotech companies and other research customers will benefit from:
- cut cost of drugs' dose-finding studies
- save time during pre-clinical phase and above
- get insights that tools of nuclear medicine cannot deliver
- reduce animal testing enormously
- new data on binding of ligends to druggable target structures
- docking at target area or in region of interest
Please find more details about the value of XFI from axiom here.